Laboratory Information Management System (LIMS) Market Trends: Automation, AI, and Cloud Innovations
United States of America– 23 Dec 2025- Laboratory Information Management Systems (LIMS) streamline lab operations by managing workflows, integrating instruments, and ensuring data security across pharmaceuticals, diagnostics, and research facilities. These platforms address rising demands for automation and compliance, enabling labs to handle complex data volumes efficiently.
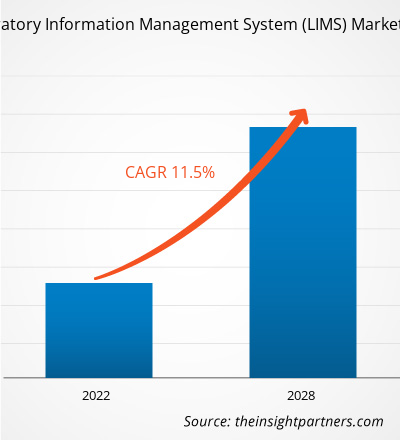
The Laboratory Information Management System (LIMS) Market was valued at US$ 1,122.36 million in 2021 and is projected to reach US$ 2,402.48 million by 2028; it is expected to grow at a CAGR of 11.5% from 2021 to 2028.
For more information-
https://www.theinsightpartners.com/reports/laboratory-information-management-systems-lims-market
Key Growth Drivers
Automation transforms routine lab tasks, reducing errors and allowing staff to focus on analysis and innovation. Labs generate vast data from high-throughput testing, fueling needs for integrated processing and real-time sharing solutions. Pharmaceutical R&D and biotech expansions amplify this, as firms prioritize data integrity for regulatory adherence like FDA standards.
Cloud-based LIMS leads deployment trends, offering scalability, remote access, and lower infrastructure costs compared to on-premises setups. These solutions support anytime data retrieval via browsers, enhancing collaboration across global teams.
Market Segmentation Insights
LIMS divides into standalone systems for isolated functions and integrated platforms combining LIMS with ELN or analytics for comprehensive oversight. Integrated options excel in complex environments, connecting facilities on cloud platforms for real-time data exchange and compliance.
For deeper analysis on segments, players, and trends, Download PDF:
https://www.theinsightpartners.com/sample/TIPHE100000954
By component, software dominates for core workflow automation, while services grow fastest through implementation, training, and maintenance support. Applications span sample tracking, logistics, and security controls, with sample management holding prominence for precise content lifecycle oversight.
End-users include hospitals, pharmaceutical firms, and contract research organizations (CROs), where CROs see rapid uptake for trial management and inventory control. Deployment favors web and cloud modes for flexibility, revolutionizing access in clinical diagnostics.
Laboratory Information Management System (LIMS) Market Trends: Automation, AI, and Cloud Innovations
United States of America– 23 Dec 2025- Laboratory Information Management Systems (LIMS) streamline lab operations by managing workflows, integrating instruments, and ensuring data security across pharmaceuticals, diagnostics, and research facilities. These platforms address rising demands for automation and compliance, enabling labs to handle complex data volumes efficiently.
The Laboratory Information Management System (LIMS) Market was valued at US$ 1,122.36 million in 2021 and is projected to reach US$ 2,402.48 million by 2028; it is expected to grow at a CAGR of 11.5% from 2021 to 2028.
For more information- https://www.theinsightpartners.com/reports/laboratory-information-management-systems-lims-market
Key Growth Drivers
Automation transforms routine lab tasks, reducing errors and allowing staff to focus on analysis and innovation. Labs generate vast data from high-throughput testing, fueling needs for integrated processing and real-time sharing solutions. Pharmaceutical R&D and biotech expansions amplify this, as firms prioritize data integrity for regulatory adherence like FDA standards.
Cloud-based LIMS leads deployment trends, offering scalability, remote access, and lower infrastructure costs compared to on-premises setups. These solutions support anytime data retrieval via browsers, enhancing collaboration across global teams.
Market Segmentation Insights
LIMS divides into standalone systems for isolated functions and integrated platforms combining LIMS with ELN or analytics for comprehensive oversight. Integrated options excel in complex environments, connecting facilities on cloud platforms for real-time data exchange and compliance.
For deeper analysis on segments, players, and trends, Download PDF: https://www.theinsightpartners.com/sample/TIPHE100000954
By component, software dominates for core workflow automation, while services grow fastest through implementation, training, and maintenance support. Applications span sample tracking, logistics, and security controls, with sample management holding prominence for precise content lifecycle oversight.
End-users include hospitals, pharmaceutical firms, and contract research organizations (CROs), where CROs see rapid uptake for trial management and inventory control. Deployment favors web and cloud modes for flexibility, revolutionizing access in clinical diagnostics.